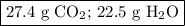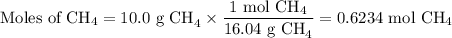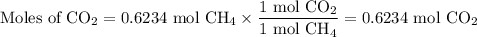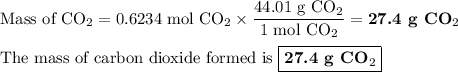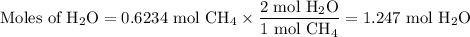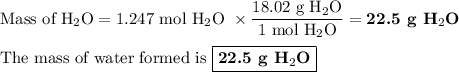Chemistry, 29.07.2019 22:20 milkshakegrande101
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left over, 27.5 grams of carbon dioxide are formed. how many grams of water are formed? ch4+ 2o2 → co2 + 2h2o

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left...
Questions

Chemistry, 07.09.2020 17:01

Social Studies, 07.09.2020 17:01

Chemistry, 07.09.2020 17:01




Medicine, 07.09.2020 17:01


Mathematics, 07.09.2020 17:01


Biology, 07.09.2020 17:01

Physics, 07.09.2020 17:01



Social Studies, 07.09.2020 17:01


Arts, 07.09.2020 17:01

Geography, 07.09.2020 17:01

Computers and Technology, 07.09.2020 17:01

Physics, 07.09.2020 17:01