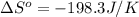Chemistry, 30.07.2019 01:10 nockturnal1993
Calculate the entropy change for the surroundings of the reaction below at 350k: n2(g) + 3h2(g) -> 2nh3(g) entropy data: nh3 = 192.5 j/mol k h2 = 130.6 j/mol k n2 = 191.5 j/mol k

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Calculate the entropy change for the surroundings of the reaction below at 350k: n2(g) + 3h2(g) -&g...
Questions

Social Studies, 11.11.2019 22:31




Mathematics, 11.11.2019 22:31



Mathematics, 11.11.2019 22:31

History, 11.11.2019 22:31



Mathematics, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31

Biology, 11.11.2019 22:31

Health, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31


Mathematics, 11.11.2019 22:31


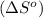 .
.
![\Delta S^o=[n_{NH_3}\times \Delta S^0_{(NH_3)}]-[n_{N_2}\times \Delta S^0_{(N_2)}+n_{H_2}\times \Delta S^0_{(H_2)}]](/tpl/images/0148/7107/be4ea.png)
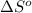 = entropy of reaction = ?
= entropy of reaction = ?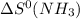 = standard entropy of
= standard entropy of 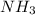
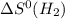 = standard entropy of
= standard entropy of 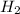
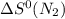 = standard entropy of
= standard entropy of 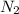
![\Delta S^o=[2mole\times (192.5J/K.mole)]-[1mole\times (191.5J/K.mole)+3mole\times (130.6J/K.mole)]](/tpl/images/0148/7107/cd252.png)