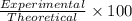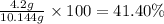Chemistry, 31.07.2019 20:40 iicekingmann
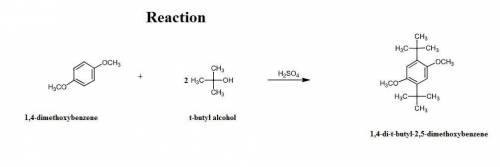
4.2 g of 1,4-di-t-butyl-2,5-dimethoxybenzene (250.37 g/mol) were synthesized by reacting 10.4 ml of t-butyl alcohol (mw 74.12 g/mol, d 0.79 g/ml), 25 ml of concentrated sulfuric acid (mw 98.08 g/mol, d 1.84 g/ml), and 5.6 g of 1,4-dimethoxybenzene (mw 138.17 g/mol) together. calculate the percent yield of this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
4.2 g of 1,4-di-t-butyl-2,5-dimethoxybenzene (250.37 g/mol) were synthesized by reacting 10.4 ml of...
Questions

Biology, 06.10.2019 10:02

Social Studies, 06.10.2019 10:02




Mathematics, 06.10.2019 10:02

Computers and Technology, 06.10.2019 10:02




Biology, 06.10.2019 10:02



Mathematics, 06.10.2019 10:02


Geography, 06.10.2019 10:02


English, 06.10.2019 10:02


Social Studies, 06.10.2019 10:02

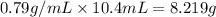
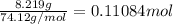

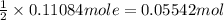 of 1,4-dimethoxybenzene.
of 1,4-dimethoxybenzene.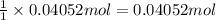 of 1,4-di-t-butyl-2,5-dimethoxybenzene.
of 1,4-di-t-butyl-2,5-dimethoxybenzene.