
Chemistry, 01.08.2019 01:20 jademckinziemea
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate constant for the reaction is 1.5 x10–2 m–1s–1, what is the concentration of a after 265 seconds? 2a --> b + crate = k[a]20.12 m0.19 m0.95 m4.0 m5.2 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate...
Questions





Mathematics, 02.03.2020 21:18

Mathematics, 02.03.2020 21:18








Physics, 02.03.2020 21:19




History, 02.03.2020 21:19


![\frac{1}{[A]}=\frac{1}{[A]_{0}}+kt](/tpl/images/0156/3924/a6d73.png)
![[A]_{0}](/tpl/images/0156/3924/48818.png) is intital concentration of A and k is rate constant.
is intital concentration of A and k is rate constant.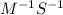 and t is 265 S
and t is 265 S![\frac{1}{[A]}=\frac{1}{0.24}+(0.015\times 265)](/tpl/images/0156/3924/91240.png)


