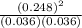Chemistry, 05.08.2019 21:20 ARAYAMYHAND
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present in a flask. the h2 and i2 react according to the balanced equation below. h2(g) + i2(g) 2 hi(g)when equilibrium is reached, the concentration of h2(g) has decreased to 0.036 mol/l. what is the equilibrium constant, kc, for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present...
Questions








Mathematics, 14.04.2021 19:40

Mathematics, 14.04.2021 19:40


Mathematics, 14.04.2021 19:40









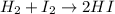
![[H_{2}]](/tpl/images/0171/8442/f6f78.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M![[I_{2}]](/tpl/images/0171/8442/d0626.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M 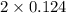
![K_{eq} = \frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0171/8442/ed373.png)