Chemistry, 06.08.2019 01:30 darius12318
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solution that is 0.682 min butanoic acid (hc4h7o2) and 0.674 min butanoate ion (c4h7o2–). calculate the ph of (a) and (b) before and after the addition of the naoh. assume volumes are additive. (ka, hc4h7o2= 1.5 × 10-5)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solu...
Questions


History, 11.07.2019 00:00

History, 11.07.2019 00:00


English, 11.07.2019 00:00

History, 11.07.2019 00:00




Mathematics, 11.07.2019 00:00


Business, 11.07.2019 00:00



English, 11.07.2019 00:00

Biology, 11.07.2019 00:00


Mathematics, 11.07.2019 00:00


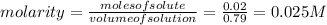
![pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0171/9988/ec35f.png)