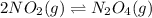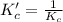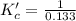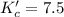The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625 mole of n2o4 was introduced into a 5.00 l vessel and was allowed to decompose until equilibrium was reached. the concentration of n2o4 at equilibrium was 0.0750 m. calculate kc for the reaction.0.0500.07500.100.1257.5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625...
Questions


Computers and Technology, 21.02.2022 06:50


Mathematics, 21.02.2022 07:00

Advanced Placement (AP), 21.02.2022 07:00


Mathematics, 21.02.2022 07:00

Mathematics, 21.02.2022 07:00

Mathematics, 21.02.2022 07:00




Mathematics, 21.02.2022 07:10

Mathematics, 21.02.2022 07:10

Mathematics, 21.02.2022 07:10

Mathematics, 21.02.2022 07:10




Chemistry, 21.02.2022 07:20

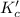 for the reaction is, 7.5
for the reaction is, 7.5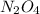 =
= 
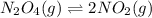
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0172/0236/271f5.png)
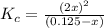
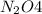 at equilibrium = 0.0750 M
at equilibrium = 0.0750 M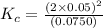
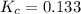
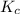 for the given reaction.
for the given reaction.