
Chemistry, 06.08.2019 02:30 jayjay9434
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the reaction starts with a mixture of pcl5, pcl3, and cl2 at pressures 0.177 atm, 0.223 atm, and 0.111 atm, respectively, at 250ºc. when the mixture comes to equilibrium at that temperature, which pressures will have decreased and which will have increased? explain why.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the re...
Questions





Mathematics, 23.07.2019 13:40

Mathematics, 23.07.2019 13:40




Social Studies, 23.07.2019 13:40

Chemistry, 23.07.2019 13:40

Mathematics, 23.07.2019 13:40








Biology, 23.07.2019 13:40

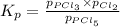
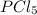 ,
, 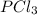 , and
, and 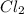 at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.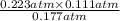
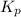 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of 

