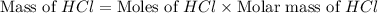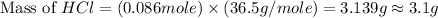Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30.0 g of hcl (fw = 36.5 g/mol) react according to the following chemical equation? mno2 + 4 hcl ® mncl2 + cl2 + 2 h2o3.1 g hcl23.3 g hcl4.02 g mno28.0 g mno212.1 g mno2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1
You know the right answer?
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30...
Questions

Computers and Technology, 13.12.2020 09:10

Mathematics, 13.12.2020 09:10




Mathematics, 13.12.2020 09:10

Mathematics, 13.12.2020 09:10



SAT, 13.12.2020 09:10




English, 13.12.2020 09:10

Mathematics, 13.12.2020 09:10

History, 13.12.2020 09:10

Mathematics, 13.12.2020 09:10


Mathematics, 13.12.2020 09:10

Computers and Technology, 13.12.2020 09:10

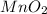 = 16.0 g
= 16.0 g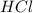 = 30.0 g
= 30.0 g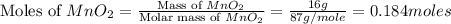
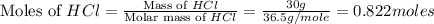
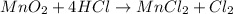
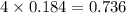 moles of
moles of