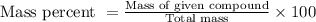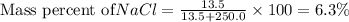Chemistry, 08.08.2019 06:10 DondreeColeman
If 13.5 g naci were dissolved in 250.0 g of water, what is the mass percent of naci in the solution? what is the mass percent of water in the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
If 13.5 g naci were dissolved in 250.0 g of water, what is the mass percent of naci in the solution?...
Questions

Biology, 06.07.2019 13:50


Social Studies, 06.07.2019 13:50

English, 06.07.2019 13:50

Business, 06.07.2019 13:50

Computers and Technology, 06.07.2019 13:50


Mathematics, 06.07.2019 13:50


Geography, 06.07.2019 13:50


English, 06.07.2019 13:50


Mathematics, 06.07.2019 13:50



Spanish, 06.07.2019 13:50



 is 6.3 % and mass percent of water is 93.7%.
is 6.3 % and mass percent of water is 93.7%.