
Chemistry, 29.01.2020 20:53 winchester729
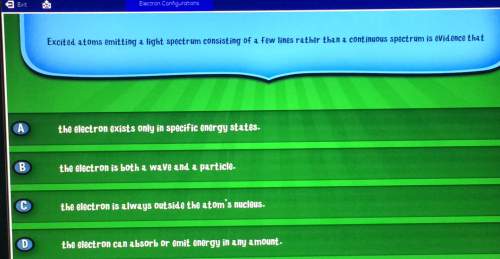
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather than a continuous spectrum is evidence thatcad the electron exists only in specific energy statesb the electron is both a wave and a particleco the electron is always outside the atom's nucleus. d the electron can absorb or emit energy in any amount

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2


Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather...
Questions

History, 26.03.2020 04:35




Computers and Technology, 26.03.2020 04:35



English, 26.03.2020 04:35


Mathematics, 26.03.2020 04:35

Mathematics, 26.03.2020 04:35

Computers and Technology, 26.03.2020 04:35


Computers and Technology, 26.03.2020 04:35



Chemistry, 26.03.2020 04:35



Mathematics, 26.03.2020 04:35



