
Chemistry, 12.08.2019 19:30 anthonylopez1
The balanced chemical equation and rate law for the reaction between no(g) and h2(g) at a particular temperature are 2no(g) + 2h2(g) → n2(g) + 2h2o(g) rate = k[no]2[h2] what is the reaction order with respect to nitric oxide? a. 4 b. 1 c. 2 d. 3 e. 0

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
The balanced chemical equation and rate law for the reaction between no(g) and h2(g) at a particular...
Questions


History, 08.03.2021 03:10


English, 08.03.2021 03:10




History, 08.03.2021 03:10



Mathematics, 08.03.2021 03:10


Computers and Technology, 08.03.2021 03:10




Mathematics, 08.03.2021 03:10

Social Studies, 08.03.2021 03:10


Biology, 08.03.2021 03:10

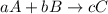
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0174/4086/10aeb.png)
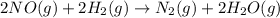
![\text{Rate}=k[NO]^2[H_2]](/tpl/images/0174/4086/3b203.png)


