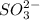Chemistry, 13.08.2019 05:30 naysia2006
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrate, and (c) potassium sulfite.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrat...
Questions

Mathematics, 10.04.2020 19:24

English, 10.04.2020 19:24

Mathematics, 10.04.2020 19:24

Biology, 10.04.2020 19:24





Chemistry, 10.04.2020 19:25









Computers and Technology, 10.04.2020 19:25

Mathematics, 10.04.2020 19:25

Mathematics, 10.04.2020 19:25

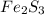
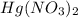
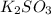
![[Ar]3d^64s^2](/tpl/images/0174/8186/7fd67.png) .
.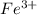 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.![[Ne]3s^23p^4](/tpl/images/0174/8186/9a0fd.png) .
.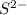 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons. ![[Xe]4f^{14}5d^{10}6s^2](/tpl/images/0174/8186/13e4d.png) .
.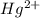 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.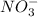
![[Ar]4s^1](/tpl/images/0174/8186/85db5.png) .
.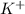 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.