
Chemistry, 18.08.2019 04:10 kadence428
The cell potential of the following electrochemical cell depends on the gold concentration in the cathode half-cell: pt(s)|h2(g,1atm)|h+(aq,1.0m)|au3+(a q,? m)|au(s). what is the concentration of au3+ in the solution if ecell is 1.27 v ? express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
The cell potential of the following electrochemical cell depends on the gold concentration in the ca...
Questions

Social Studies, 21.09.2019 10:30



Arts, 21.09.2019 10:30

Mathematics, 21.09.2019 10:30



Mathematics, 21.09.2019 10:30


Biology, 21.09.2019 10:30




History, 21.09.2019 10:30

Mathematics, 21.09.2019 10:30

History, 21.09.2019 10:30

Biology, 21.09.2019 10:30



Mathematics, 21.09.2019 10:30

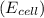 is given as 1.27 V.
is given as 1.27 V.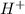 = 1.0 M
= 1.0 M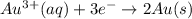
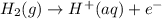
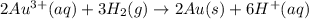
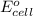 ) for hydrogen is equal to zero.
) for hydrogen is equal to zero.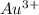 is
is  equal 1.50 V.
equal 1.50 V.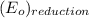 -
- 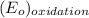
![E_{cell} = E^{o}_{cell} - \frac{0.059}{2}log \frac{[H^{+}]^{6}}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/b4490.png)
![log\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/7ec17.png) = 23
= 23![\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/cebd5.png) =
= 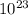
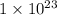
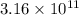
![[Au^{3+}]](/tpl/images/0175/5466/7b483.png) =
= 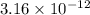 M
M

