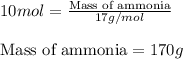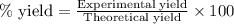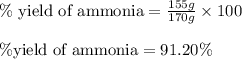Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g) + 3h2 (g) 2nh3 (g). if 140 grams of nitrogen gas is allowed to react with an excess of hydrogen gas to produce 155 grams of ammonia, what is the percent yield of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g...
Questions


Computers and Technology, 28.10.2020 21:20



Health, 28.10.2020 21:20

Mathematics, 28.10.2020 21:20

English, 28.10.2020 21:20

Mathematics, 28.10.2020 21:20

Mathematics, 28.10.2020 21:20

Mathematics, 28.10.2020 21:20



Biology, 28.10.2020 21:20




Mathematics, 28.10.2020 21:20


Mathematics, 28.10.2020 21:20

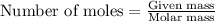
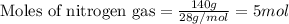
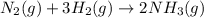
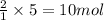 of ammonia.
of ammonia.