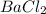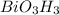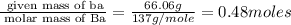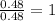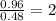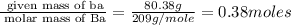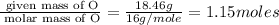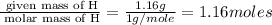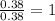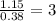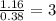Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
Determine the empirical formula for compounds that have the following analyses: a. 66.0% barium and...
Questions


Arts, 23.01.2020 05:31


Health, 23.01.2020 05:31

Business, 23.01.2020 05:31









Social Studies, 23.01.2020 05:31