Chemistry, 30.08.2019 16:10 derbraz6770
A250 cm^3 solution containing 1,46 g of sodium chloride is added to an excess of silver nitrate solution. the reaction is given. nacl (aq)+agno, (aq)-agci(s)+nano, (aq) what is the concentration of the sodium chloride solution? (4) 7.1 calculate the mass of the precipitate. 7.2 (4) 18

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
A250 cm^3 solution containing 1,46 g of sodium chloride is added to an excess of silver nitrate solu...
Questions





English, 17.01.2020 19:31




Chemistry, 17.01.2020 19:31


Computers and Technology, 17.01.2020 19:31









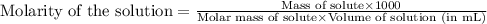
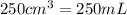
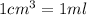
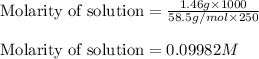
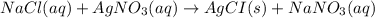
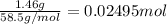
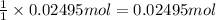 of AgCl
of AgCl