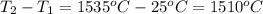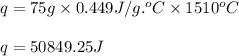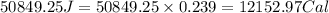Chemistry, 03.09.2019 01:10 xeskimopie
How much heat, in joules and in calories, must be added to a 75.0- g iron block with a specific heat of 0.449 j/g degree cecius to increas its temperature from 25 degree celcius to its melting temperature of 1535 dgree celcius?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
How much heat, in joules and in calories, must be added to a 75.0- g iron block with a specific heat...
Questions


Biology, 19.03.2021 07:40

Mathematics, 19.03.2021 07:40


Chemistry, 19.03.2021 07:40

Mathematics, 19.03.2021 07:40



Mathematics, 19.03.2021 07:40

Mathematics, 19.03.2021 07:40


Mathematics, 19.03.2021 07:40

Mathematics, 19.03.2021 07:40





History, 19.03.2021 07:40


Mathematics, 19.03.2021 07:50

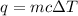
 = change in temperature =
= change in temperature =