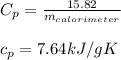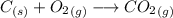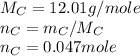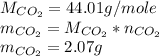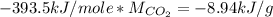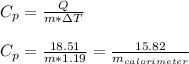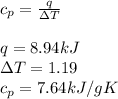Asample of 0.5662 g of carbon is burned in oxygen in a bomb calrimeter, producing carbon dioxide, assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy of combustion of graphite. the temperature of the calorimeter increases from 26.74c to 27.93c. what is the heat capacity of the calorimeter and its contents?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Asample of 0.5662 g of carbon is burned in oxygen in a bomb calrimeter, producing carbon dioxide, as...
Questions

Biology, 28.08.2019 16:40

History, 28.08.2019 16:40




Health, 28.08.2019 16:40

Mathematics, 28.08.2019 16:40

Social Studies, 28.08.2019 16:40





Mathematics, 28.08.2019 16:40

Mathematics, 28.08.2019 16:40

History, 28.08.2019 16:40


Computers and Technology, 28.08.2019 16:40

Biology, 28.08.2019 16:40

Mathematics, 28.08.2019 16:40

Social Studies, 28.08.2019 16:40