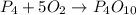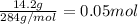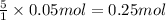Chemistry, 04.09.2019 05:20 vannybelly83
Given the unbalanced reaction: p₄+ o₂  p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
a. 0.125 mol
b. 0.0620 mol
c. 0.500 mol
d. 0.0500 mol
e. 0.250 mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Given the unbalanced reaction: p₄+ o₂ [tex]\rightarrow[/tex] p₄o₁₀ how many moles of o₂ are needed...
Questions



History, 22.02.2021 18:40

Social Studies, 22.02.2021 18:40


English, 22.02.2021 18:40

Mathematics, 22.02.2021 18:40

Mathematics, 22.02.2021 18:40


English, 22.02.2021 18:40

Mathematics, 22.02.2021 18:40


Mathematics, 22.02.2021 18:40

Mathematics, 22.02.2021 18:40