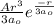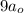Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
34. an electron in the 3d state of the hydrogen atom has a radial part of the wave function of the f...
Questions


Mathematics, 12.11.2020 02:10




Mathematics, 12.11.2020 02:10


History, 12.11.2020 02:10

Computers and Technology, 12.11.2020 02:10



Mathematics, 12.11.2020 02:10



Mathematics, 12.11.2020 02:10



Computers and Technology, 12.11.2020 02:10



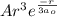
 = 0
= 0 +
+ 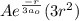 = 0
= 0