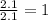Measurements show that unknown compound x has the following composition:
element mass %
...

Chemistry, 05.09.2019 17:10 samsmith666
Measurements show that unknown compound x has the following composition:
element mass %
carbon 62.4
hydrogen 4.19
oxygen 33.2
write the empirical chemical formula of x?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

You know the right answer?
Questions

History, 08.02.2021 17:10


Mathematics, 08.02.2021 17:10

History, 08.02.2021 17:10



French, 08.02.2021 17:10

Mathematics, 08.02.2021 17:10



Mathematics, 08.02.2021 17:10

Biology, 08.02.2021 17:10

Mathematics, 08.02.2021 17:10


Chemistry, 08.02.2021 17:10

World Languages, 08.02.2021 17:10

World Languages, 08.02.2021 17:10

Mathematics, 08.02.2021 17:10


 .
.