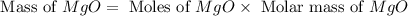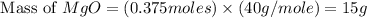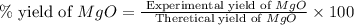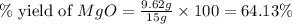Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced equation for the reaction is 2 mg(s) + o2(g) → 2 mgo(s) now consider that you react 10.0 g mg with 6.00 g o2 gas. if you were able to collect 9.62 g of mgo, what would be your percent yield for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3

Chemistry, 23.06.2019 13:30
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1

You know the right answer?
Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced e...
Questions






Mathematics, 03.07.2019 00:00


Mathematics, 03.07.2019 00:00



English, 03.07.2019 00:00

English, 03.07.2019 00:00

Mathematics, 03.07.2019 00:00



Biology, 03.07.2019 00:00


Mathematics, 03.07.2019 00:00


Biology, 03.07.2019 00:00

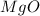 is, 64.13 %
is, 64.13 %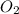 = 6 g
= 6 g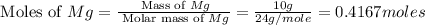
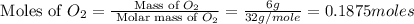
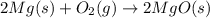
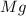
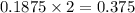 moles of
moles of