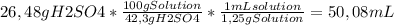Chemistry, 09.09.2019 19:10 runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions


Mathematics, 08.11.2019 23:31

History, 08.11.2019 23:31

Biology, 08.11.2019 23:31


History, 08.11.2019 23:31


Mathematics, 08.11.2019 23:31

English, 08.11.2019 23:31


Mathematics, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31

Biology, 08.11.2019 23:31


Business, 08.11.2019 23:31

History, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31