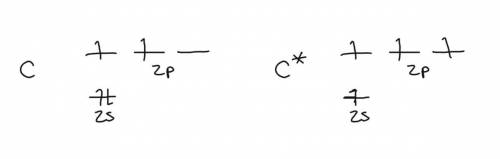Chemistry, 10.09.2019 21:30 sriharin58ozhj9m
(a) consider a carbon atom in its ground state. would such an atom offer a satisfactory model for the carbon of methane? if not, why not? (hint: consider whether a ground state carbon atom could be tetravalent, and consider the bond angles that would result if it were to combine with hydrogen atoms.)
(b) consider a carbon atom in the excited state. would such an atom offer a satisfactory model for the carbon of methane? if not, why not?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
(a) consider a carbon atom in its ground state. would such an atom offer a satisfactory model for th...
Questions

English, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30





Mathematics, 19.07.2019 23:30

History, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30






Mathematics, 19.07.2019 23:30

Health, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30