
Chemistry, 12.09.2019 20:30 Pointjazzyqueen602
An aerosol can with a temperature of 292 k and a pressure of 1.25 atm was left outside in the sun on a hot summer day. the pressure inside the can rose to 1.33 atm. what was the resulting temperature in the can?
322k
1,600k
311k
274k
ik its not a

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
An aerosol can with a temperature of 292 k and a pressure of 1.25 atm was left outside in the sun on...
Questions


Mathematics, 20.06.2020 20:57




Biology, 20.06.2020 20:57

Social Studies, 20.06.2020 20:57









Mathematics, 20.06.2020 20:57




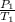 =
= 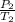
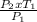
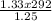 = 310.69K
= 310.69K

