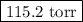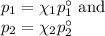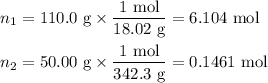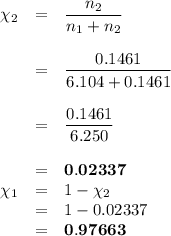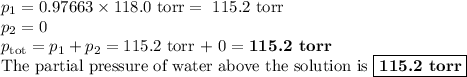Chemistry, 12.09.2019 21:30 pegflans314
Asolution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the partial pressure of water above the solution is torr. the vapor pressure of pure water at 55 °c is 118.0 torr. the mw of lactose is 342.3 g/mol. a solution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the partial pressure of water above the solution is torr. the vapor pressure of pure water at 55 °c is 118.0 torr. the mw of lactose is 342.3 g/mol. 156.8 2.757 115.2 282.3 81.1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Asolution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the pa...
Questions



Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20





English, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20



Computers and Technology, 10.05.2021 23:20



Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20