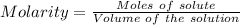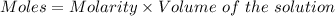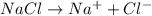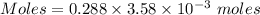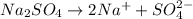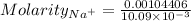Chemistry, 13.09.2019 22:30 jazz589729
Calculate the molarity of sodium ion in a solution made
bymixing 3.58 ml of 0.288 m sodium chloride with 500 ml of 6.51
times1/1000 m sodium sulfate ( assume volumes are additive ).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Calculate the molarity of sodium ion in a solution made
bymixing 3.58 ml of 0.288 m sodium chl...
bymixing 3.58 ml of 0.288 m sodium chl...
Questions


Mathematics, 23.03.2021 04:50



Computers and Technology, 23.03.2021 04:50

Mathematics, 23.03.2021 04:50

Biology, 23.03.2021 04:50





Mathematics, 23.03.2021 04:50

Social Studies, 23.03.2021 04:50




History, 23.03.2021 04:50