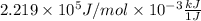Chemistry, 14.09.2019 09:30 victoriadorvilu
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increases to 600. mm hg at 1220.°c. determine the molar heat of vaporization of substance x using the derived form of the clausius-clapeyron equation given below. (include the sign of the value in your answer.) kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increa...
Questions


Computers and Technology, 14.01.2021 23:10



Mathematics, 14.01.2021 23:10






Mathematics, 14.01.2021 23:10

Social Studies, 14.01.2021 23:10


Health, 14.01.2021 23:10



Mathematics, 14.01.2021 23:10



Mathematics, 14.01.2021 23:10

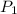 = 100 mm Hg or
= 100 mm Hg or 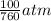 = 0.13157 atm
= 0.13157 atm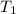 =
= 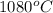 = (1080 + 273) K = 1357 K
= (1080 + 273) K = 1357 K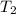 =
= 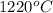 = (1220 + 273) K = 1493 K
= (1220 + 273) K = 1493 K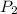 = 600 mm Hg or
= 600 mm Hg or 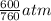 = 0.7895 atm
= 0.7895 atm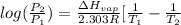
![log(\frac{0.7895}{0.13157}) = \frac{\Delta H_{vap}}{2.303 \times 8.314 J/mol K}[\frac{1}{1357 K} - \frac{1}{1493 K}]](/tpl/images/0231/2242/21278.png)
![log (6) = \frac{\Delta H_{vap}}{19.147}[\frac{(1493 - 1357) K}{1493 K \times 1357 K}]](/tpl/images/0231/2242/46d38.png)
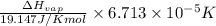
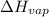 =
= 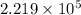 J/mol
J/mol