
Chemistry, 18.09.2019 04:00 Elepeodowke
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 × 10−4 g mgcl2 in 2.252.25 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 ×...
Questions



Computers and Technology, 04.07.2019 22:30


Biology, 04.07.2019 22:30

World Languages, 04.07.2019 22:30







Mathematics, 04.07.2019 22:30


Mathematics, 04.07.2019 22:30


Mathematics, 04.07.2019 22:30



Computers and Technology, 04.07.2019 22:30

 are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.
are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively. =
= 


 and
and 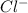 .
.
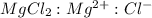 is, 1 : 1 : 2. So,
is, 1 : 1 : 2. So,

