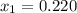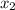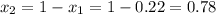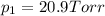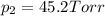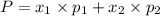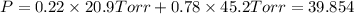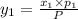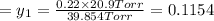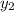Chemistry, 20.09.2019 03:00 aljalloh94
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in all proportions. let x1 and x2 represent the mole fractions of 1‑propanol and 2‑propanol in a liquid mixture, respectively, and y1 and y2 represent the mole fractions of each in the vapor phase. for a solution of these liquids with x1=0.220, calculate the composition of the vapor phase at 25 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

You know the right answer?
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in...
Questions







Mathematics, 05.05.2020 16:44

Biology, 05.05.2020 16:44




History, 05.05.2020 16:44





Mathematics, 05.05.2020 16:44