
Be sure to answer all parts. consider the reaction a + b → products from the following data obtained at a certain temperature, determine the order of the reaction. enter the order with respect to a, the order with respect to b, and the overall reaction order. a 0.213 [a] (m) [b] (m) rate (m/s) 1.50 1.50 3.20 × 10−1 1.50 2.50 3.20 × 10−1 3.00 1.50 6.40 × 10−1 b 0.213 reaction 1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
Be sure to answer all parts. consider the reaction a + b → products from the following data obtained...
Questions



Mathematics, 12.03.2020 01:59



Mathematics, 12.03.2020 01:59



Mathematics, 12.03.2020 01:59

Physics, 12.03.2020 01:59




History, 12.03.2020 01:59






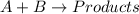
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0265/2010/10aeb.png)
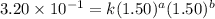 ....(1)
....(1)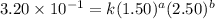 ....(2)
....(2)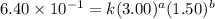 ....(3)
....(3)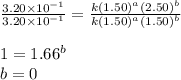
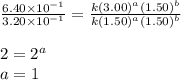
![\text{Rate}=k[A]^1[B]^0](/tpl/images/0265/2010/de6a4.png)
![\text{Rate}=k[A]](/tpl/images/0265/2010/660c6.png)


