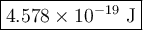Chemistry, 28.09.2019 23:10 zacksoccer8279
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission spectrum has a violet, a blue, a teal, and a red line. calculate the energy of one photon of this light.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission sp...
Questions


Mathematics, 29.09.2020 01:01









Social Studies, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01






History, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01