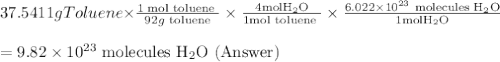One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 43.3 ml of toluene (d = 0.867 g/ml) is consumed when a sample of gasoline burns in air. how many grams of oxygen are needed for complete combustion of the toluene? (a) how many grams of oxygen are needed for complete combustion of the toluene? g (b) how many total moles of gaseous products form? mol (c) how many molecules of water vapor form?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 22:30
Acompound consisting of b, n, and h undergoes elemental analysis. the % composition by mass is found to be 40.28% b, 52.20% n, and 7.53% h. what is the empirical formula for this molecule? enter in the empirical formula in the form bxnyhz
Answers: 1
You know the right answer?
One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 4...
Questions


Mathematics, 28.07.2019 22:30


Mathematics, 28.07.2019 22:30

Arts, 28.07.2019 22:30

History, 28.07.2019 22:30



Biology, 28.07.2019 22:30

Chemistry, 28.07.2019 22:30


Mathematics, 28.07.2019 22:30

Social Studies, 28.07.2019 22:30

English, 28.07.2019 22:30


Mathematics, 28.07.2019 22:30

Physics, 28.07.2019 22:30



History, 28.07.2019 22:30

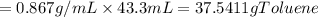
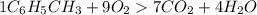
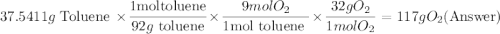
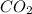 gas and 4 moles of
gas and 4 moles of 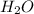 Vapour
Vapour
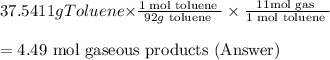
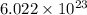 molecules
molecules