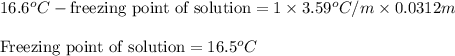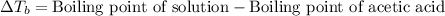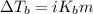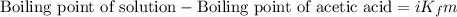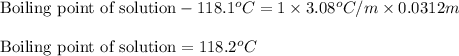Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1


Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
You know the right answer?
Asolution was prepared by dissolving 0.800 g of sulfur s8, in 100.0 g of acetic acid, hc2h3o2. calcu...
Questions



English, 21.12.2020 14:00

History, 21.12.2020 14:00


English, 21.12.2020 14:00


Health, 21.12.2020 14:00



Chemistry, 21.12.2020 14:00

Geography, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Social Studies, 21.12.2020 14:00

Social Studies, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

English, 21.12.2020 14:00


Mathematics, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

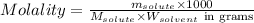
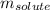 = Given mass of solute
= Given mass of solute 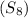 = 0.800 g
= 0.800 g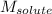 = Molar mass of solute
= Molar mass of solute  = 256.52 g/mol
= 256.52 g/mol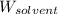 = Mass of solvent (acetic acid) = 100.0 g
= Mass of solvent (acetic acid) = 100.0 g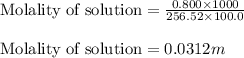
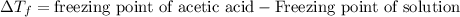
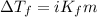
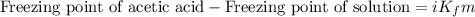
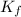 = molal freezing point depression constant = 3.59°C/m
= molal freezing point depression constant = 3.59°C/m