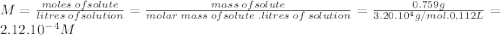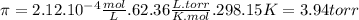Chemistry, 02.10.2019 22:00 veronica25681
Astarch has a molar mass of 3.20 x 104 g/mol. if 0.759 g of this starch is dissolved in 112 ml of solution, what is the osmotic pressure, in torr, at 25.00 oc?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Astarch has a molar mass of 3.20 x 104 g/mol. if 0.759 g of this starch is dissolved in 112 ml of so...
Questions



Mathematics, 22.04.2020 23:31

History, 22.04.2020 23:31

Mathematics, 22.04.2020 23:31

Biology, 22.04.2020 23:31










Mathematics, 22.04.2020 23:31