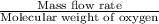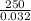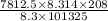Chemistry, 03.10.2019 02:00 alannaswitzer
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to estimate the volumetric flow rate (l/hr) of this stream. (see example 5.3-3.) the ideal gas equation of state is an approximation. under which conditions, it is suggested that the ideal gas equation be used for? select one: o a. temperatures above about 0°c and pressures below about 1 atm o b. temperatures below about 0°c and pressures below about 1 atm c. temperatures above about 0°c and pressures above about 1 atm d. under any condition e. standard condition of 25°c and 1 atm o o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to...
Questions


Medicine, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01

French, 26.08.2020 22:01

Spanish, 26.08.2020 22:01

English, 26.08.2020 22:01


History, 26.08.2020 22:01

Physics, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01

English, 26.08.2020 22:01

History, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01



Mathematics, 26.08.2020 22:01


History, 26.08.2020 22:01