
Chemistry, 03.10.2019 05:20 aaronw3743
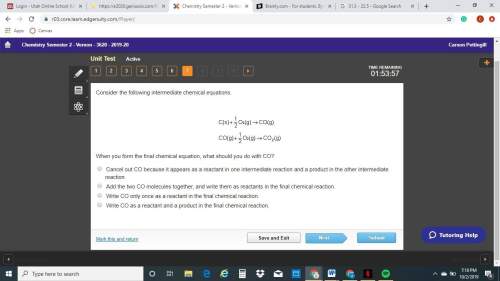
When you form the final chemical equation, what should you do with co? cancel out co because it appears as a reactant in one intermediate reaction and a product in the other intermediate reaction. add the two co molecules together, and write them as reactants in the final chemical reaction. write co only once as a reactant in the final chemical reaction. write co as a reactant and a product in the final chemical reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
When you form the final chemical equation, what should you do with co? cancel out co because it app...
Questions



Social Studies, 12.10.2020 19:01



Mathematics, 12.10.2020 19:01

Geography, 12.10.2020 19:01


English, 12.10.2020 19:01

History, 12.10.2020 19:01

History, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Social Studies, 12.10.2020 19:01



Mathematics, 12.10.2020 19:01

Biology, 12.10.2020 19:01



Mathematics, 12.10.2020 19:01



