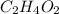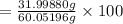Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Acetic acid (hc2h3o2) is the active ingredient in vinegar. calculate the mass percent composition of...
Questions


Health, 28.04.2021 02:10

Spanish, 28.04.2021 02:10



History, 28.04.2021 02:10


History, 28.04.2021 02:10




Spanish, 28.04.2021 02:10

English, 28.04.2021 02:10


Spanish, 28.04.2021 02:10

Arts, 28.04.2021 02:10

Chemistry, 28.04.2021 02:10



Mathematics, 28.04.2021 02:20