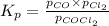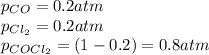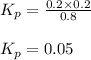Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, previously evacuated flask at 690 k, the pressure in the flask is initially 1.0 atm. after the reaction reaches equilibrium at 690 k, the total pressure in the flask is 1.2 atm. what is the value of kp for the reaction at 690 k?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, pr...
Questions




Mathematics, 25.08.2019 13:30

Mathematics, 25.08.2019 13:30




Mathematics, 25.08.2019 13:30


Mathematics, 25.08.2019 13:30

Mathematics, 25.08.2019 13:30

Biology, 25.08.2019 13:30



Mathematics, 25.08.2019 13:30


English, 25.08.2019 13:30

Mathematics, 25.08.2019 13:30

Biology, 25.08.2019 13:30

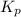 for the reaction at 690 K is 0.05
for the reaction at 690 K is 0.05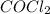 = 1.0 atm
= 1.0 atm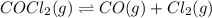
![[(1 - x) + x+ x]=1.2\\\\x=0.2atm](/tpl/images/0292/9648/696d1.png)