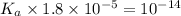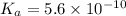Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2


Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
You know the right answer?
Given that at 25.0 ∘c ka for hcn is 4.9×10−10 and kb for nh3 is 1.8×10−5, calculate kb for cn− and k...
Questions

History, 12.08.2020 05:01




Mathematics, 12.08.2020 05:01




Health, 12.08.2020 05:01



Physics, 12.08.2020 05:01

Biology, 12.08.2020 05:01

History, 12.08.2020 05:01

Physics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01

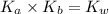
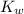 is the dissociation constant of water.
is the dissociation constant of water.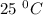 ,
, 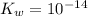
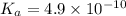
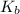 for CN⁻ can be calculated as:
for CN⁻ can be calculated as:
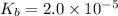
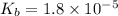
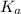 for
for 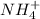 can be calculated as:
can be calculated as: