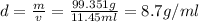Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
When a 25.00 ml volumetric flask weighing 20.340 g is filled partially with metal shot the mass is 1...
Questions



Mathematics, 23.04.2020 00:41


Mathematics, 23.04.2020 00:41

Mathematics, 23.04.2020 00:41


Advanced Placement (AP), 23.04.2020 00:41

History, 23.04.2020 00:41


Mathematics, 23.04.2020 00:42


English, 23.04.2020 00:42



Physics, 23.04.2020 00:43



Mathematics, 23.04.2020 00:43

Spanish, 23.04.2020 00:43

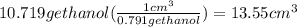 or 13.55 ml of ethanol
or 13.55 ml of ethanol