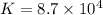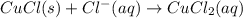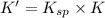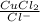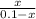The copper(I) ion forms a chloride salt (CuCl) that has Ksp = 1.2 x 10-6. Copper(I) also forms a complex ion with Cl-:Cu+ (aq) + 2Cl- (aq) ⇄ CuCl2- (aq) K = 8.7 x 104(a) Calculate the solubility of CuCl in pure water. (Ignore CuCl2- formation for part a).(b) Calculate the solubility of CuCl in 0.100 M NaCl solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
The copper(I) ion forms a chloride salt (CuCl) that has Ksp = 1.2 x 10-6. Copper(I) also forms a com...
Questions


Geography, 13.08.2020 18:01

History, 13.08.2020 18:01



History, 13.08.2020 18:01

Mathematics, 13.08.2020 18:01

Biology, 13.08.2020 18:01

Mathematics, 13.08.2020 18:01




Mathematics, 13.08.2020 18:01





History, 13.08.2020 18:01

Mathematics, 13.08.2020 18:01

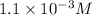 .
.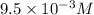 .
.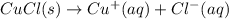
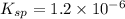
![K_{sp} = [Cu^{+}][Cl^{-}]](/tpl/images/0619/9671/6e8cf.png)
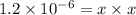
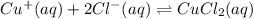 ,
,