
Chemistry, 09.10.2019 01:00 jtaylor4061
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.10 mole of powdered silver is added to 10.0 milliliters of 6.0 molar nitric acid, the number of moles of no gas that can be formed is
a) 0.015 mole
b) 0.020 mole
c) 0.030 mole
d) 0.045 mole
e) 0.090 mole

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.1...
Questions

Mathematics, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


Health, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


History, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00


English, 07.07.2019 17:00

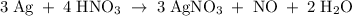
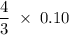 moles nitric acid
moles nitric acid

