Chemistry, 10.10.2019 22:10 BaileyElizabethRay
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is ca(oh)2(s) + co2(g) → caco3(s) + h2o(g) δh = –69.1 kj what is the enthalpy change if 3.8 mol of calcium carbonate is formed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions

English, 31.01.2021 08:20

Mathematics, 31.01.2021 08:20



English, 31.01.2021 08:20

Business, 31.01.2021 08:20


Mathematics, 31.01.2021 08:20



Biology, 31.01.2021 08:20

History, 31.01.2021 08:20

English, 31.01.2021 08:20

Engineering, 31.01.2021 08:20


Health, 31.01.2021 08:20


Mathematics, 31.01.2021 08:20

Mathematics, 31.01.2021 08:20


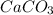 are formed.
are formed.