
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the reaction is 1.94 × 10-4 min-1. if the initial pressure of n2o is 4.70 atm at 730°c, calculate the total gas pressure after one half-life. assume that the volume remains constant. slatter

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the...
Questions





Mathematics, 16.10.2020 18:01






Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

English, 16.10.2020 18:01


English, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01

History, 16.10.2020 18:01

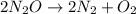
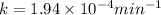
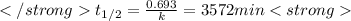
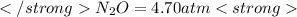
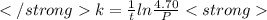
 after first half life = 2.35 = 4.70 - 2x
after first half life = 2.35 = 4.70 - 2x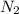 after first half life = 2x = 2(1.175) = 2.35 ATM
after first half life = 2x = 2(1.175) = 2.35 ATM

