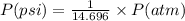Chemistry, 15.10.2019 20:20 rissaroo159
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the tire is 860 ml , that it is filled to a total pressure of 120 psi , and that the temperature is 26 ∘c. also, assume an average molar mass for air of 28.8 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

You know the right answer?
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the ti...
Questions

English, 04.06.2020 19:01

Mathematics, 04.06.2020 19:01

Mathematics, 04.06.2020 19:01



History, 04.06.2020 19:01

Mathematics, 04.06.2020 19:01

Mathematics, 04.06.2020 19:01

Computers and Technology, 04.06.2020 19:01






English, 04.06.2020 19:01



Mathematics, 04.06.2020 19:02