
The automobile fuel called e85 consists of 85% ethanol and 15% gasoline. e85 can be used in so-called flex-fuel vehicles (ffvs), which can use gasoline, ethanol, or a mix as fuels. assume that gasoline consists of a mixture of octanes (different isomers of c₈h₁₈), that the average heat of combustion of c₈h₁₈(l) is 5400 kj/mol, and that gasoline has an average density of 0.70 g/ml. the density of ethanol is 0.79 g/ml. by using the information given, calculate the energy produced by combustion of 1.5 l of gasoline. express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
The automobile fuel called e85 consists of 85% ethanol and 15% gasoline. e85 can be used in so-calle...
Questions




Mathematics, 22.11.2019 06:31




Mathematics, 22.11.2019 06:31

English, 22.11.2019 06:31


Mathematics, 22.11.2019 06:31




Mathematics, 22.11.2019 06:31

Biology, 22.11.2019 06:31



Mathematics, 22.11.2019 06:31

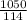 = 9.21 mol
= 9.21 mol

