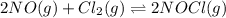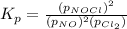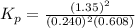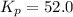Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
The partial pressures in an equilibrium mixture of no, cl2, and noci at 500 k are as follows: pno =...
Questions

Mathematics, 03.09.2020 23:01



Mathematics, 03.09.2020 23:01



Mathematics, 03.09.2020 23:01

History, 03.09.2020 23:01



Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01


Physics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01



Social Studies, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

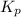 at temperature 500 K is 52.0
at temperature 500 K is 52.0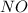 at equilibrium = 0.240 atm
at equilibrium = 0.240 atm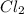 at equilibrium = 0.608 atm
at equilibrium = 0.608 atm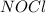 at equilibrium = 1.35 atm
at equilibrium = 1.35 atm