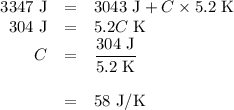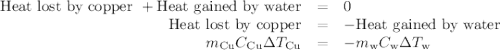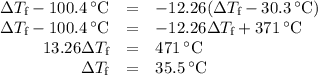Chemistry, 18.10.2019 17:10 tonimgreen17p6vqjq
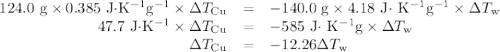
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is heated to 100.4°c by putting it in a beaker of boiling water. the specific heat of cu(s) is 0.385 j/g⋅k. the cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.3°c .
(a)- determine the amount of heat, in j, lost by the copper block. enter the absolute amount of heat lost by cu, without a minus sign.
(b)- determine the amount of heat gained by the water. the specific heat of water is 4.18 j/g⋅k.
(c)-the difference between your answers for (a) and (b) is due to heat loss through the styrofoam → cups and the heat necessary to raise the temperature of the inner wall of the apparatus. the heat
capacity of the calorimeter is the amount of heat necessary to raise the temperature of the apparatus (the cups and the stopper) by 1 k. calculate the heat capacity of the calorimeter in j/k.
expressyour answer using two significant figures.
(d)-what would be the final temperature of the system if all the heat lost by the copper block were absorbed by the water in the calorimeter?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is hea...
Questions


Mathematics, 05.05.2020 03:23

Advanced Placement (AP), 05.05.2020 03:23


Mathematics, 05.05.2020 03:23

Chemistry, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23




Mathematics, 05.05.2020 03:23

Social Studies, 05.05.2020 03:23

Physics, 05.05.2020 03:23



Mathematics, 05.05.2020 03:23


Mathematics, 05.05.2020 03:23